publications
2025
- JACC Adv.
 Machine learning reconstruction of left ventricular pressure from peripheral waveformsAlessio Tamborini, Arian Aghilinejad, Ray V Matthews, and 1 more authorJACC Advances, Aug 2025
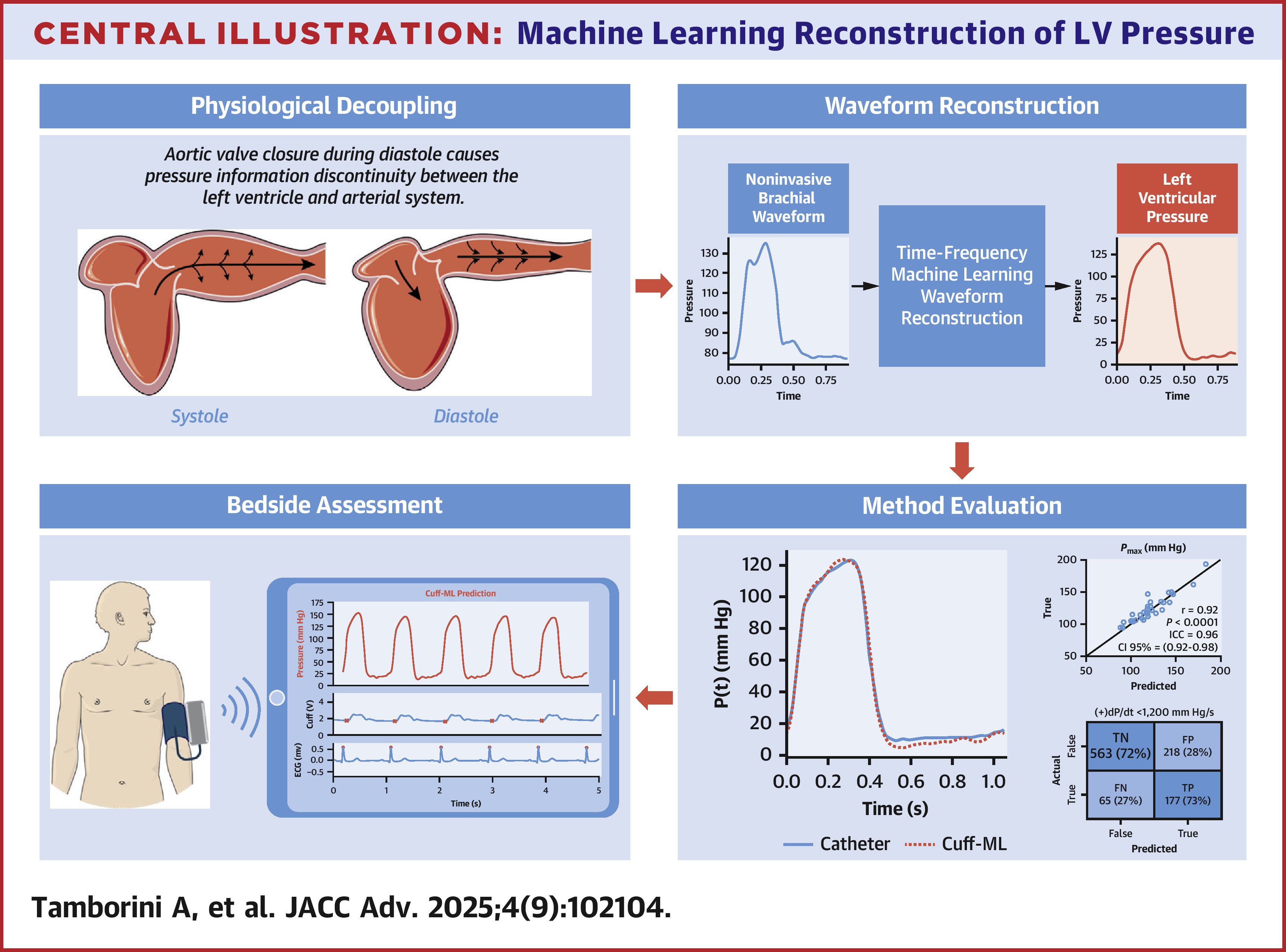
Machine learning reconstruction of left ventricular pressure from peripheral waveformsAlessio Tamborini, Arian Aghilinejad, Ray V Matthews, and 1 more authorJACC Advances, Aug 2025BACKGROUND: Left ventricular (LV) pressure measurement is the clinical gold standard for assessing cardiac function; however, its reliance on invasive catheterization limits accessibility and widespread use. OBJECTIVES: This study aimed to develop a cuff-based machine learning (cuff-ML) approach for reconstructing LV pressure from noninvasive brachial waveforms as a bedside assessment of cardiac function. METHODS: Subjects referred for nonemergent left heart catheterization were recruited for LV pressure and brachial cuff waveform measurement. The cuff-ML method was trained using brachial waveforms to predict LV pressure and was evaluated for morphology and parameters accuracy against invasive catheter measurements. Cardiac function was assessed based on the reduced LV peak pressure derivative ([+]dP/dt <1,200 mm Hg/s). RESULTS: A total of 104 subjects, comprising 3,572 simultaneous LV and cuff-based brachial waveform pairs, were analyzed using a 70:30 train-test split (test cohort: 32 subjects, 1,023 cardiac cycles). The cuff-ML approach demonstrated high accuracy in reconstructing LV waveform shape compared to catheter measurements (median normalized root mean squared error = 8.2%). Pressure-based parameters, including maximum pressure (r = 0.92, P < 0.001), mean blood pressure (r = 0.94, P < 0.001), and developed pressure (r = 0.85, P < 0.001), showed strong correlations with invasive measurements. Cuff-ML-reconstructed waveforms identified abnormal systolic contractility (72% sensitivity, 73% specificity) on a beat-to-beat basis. CONCLUSIONS: Cuff-ML accurately reconstructs LV pressure from brachial cuff measurements. This noninvasive approach may be helpful for assessment of cardiac function and requires further study.
- Patent
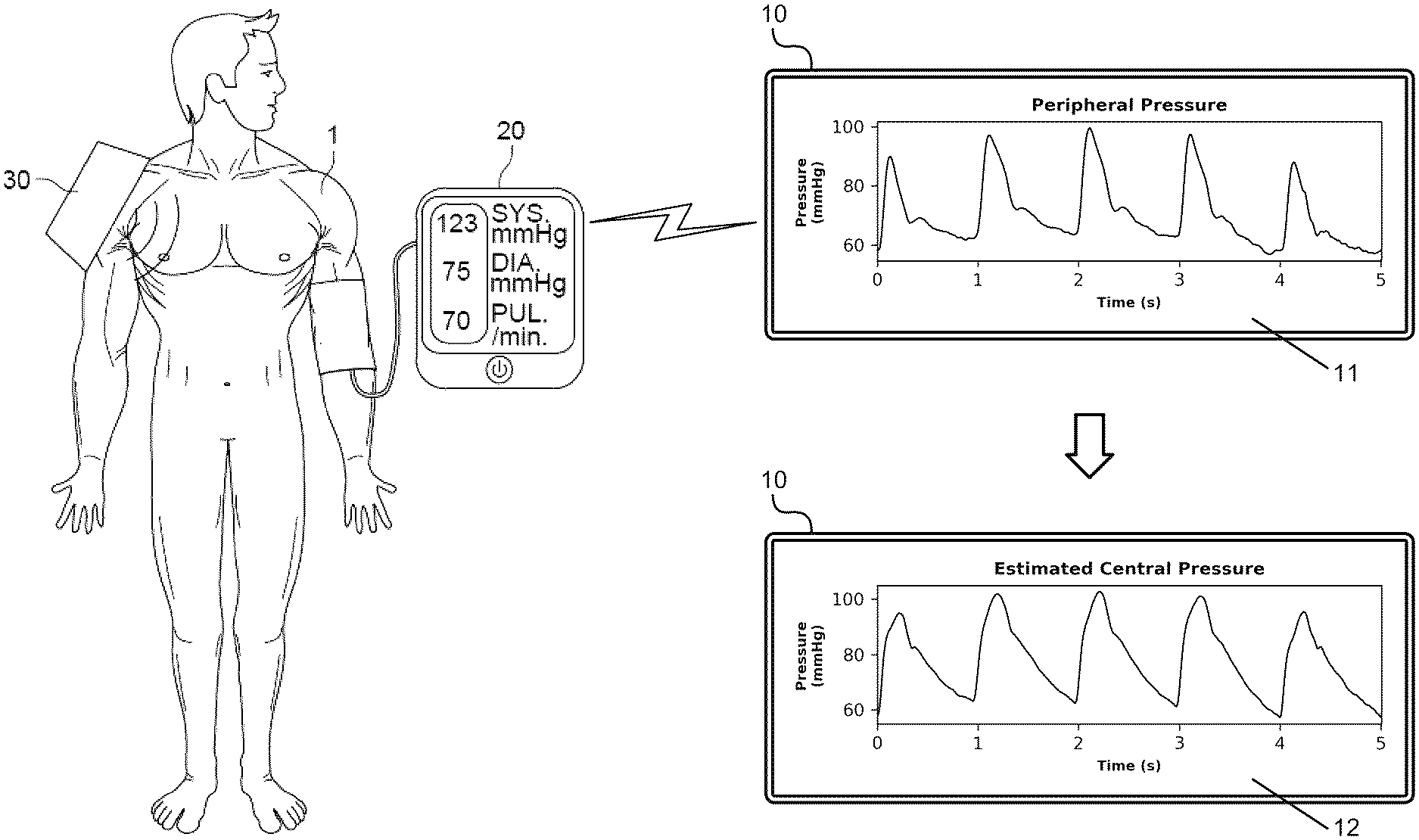 Method for transferring a pressure wave from peripheral site to central siteAlessio Tamborini, Morteza Gharib, and Arian AghilinejadUS Patent US20250082280A1, Mar 2025
Method for transferring a pressure wave from peripheral site to central siteAlessio Tamborini, Morteza Gharib, and Arian AghilinejadUS Patent US20250082280A1, Mar 2025 - PNAS
 A spectral machine learning approach to derive central aortic pressure waveforms from a brachial cuffAlessio Tamborini, Arian Aghilinejad, and Morteza GharibProceedings of the National Academy of Sciences, Mar 2025
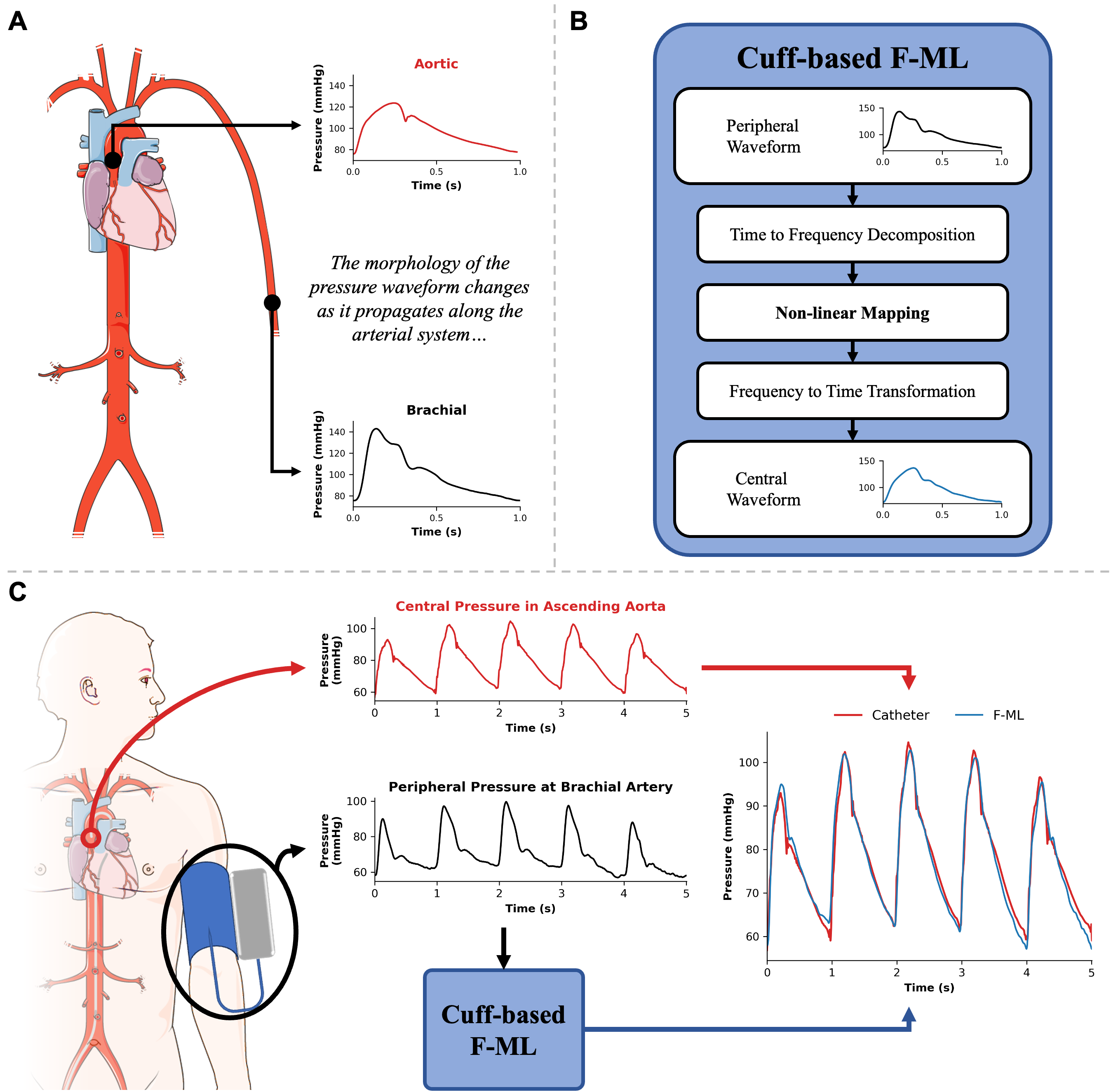
A spectral machine learning approach to derive central aortic pressure waveforms from a brachial cuffAlessio Tamborini, Arian Aghilinejad, and Morteza GharibProceedings of the National Academy of Sciences, Mar 2025Mapping peripheral to central pressure waveforms offers a promising approach for noninvasive assessment of the aortic pressure waveform. Traditional methods rely on population-based averaging, which fail to account for the complex wave interactions within the arterial system, leading to poor morphological accuracy. This study introduces a wave-based approach that combines brachial cuff measurements with machine learning to nonlinearly map brachial wave components to the aortic site, enabling accurate reconstruction of the central pressure waveform. By accounting for nonlinear wave interactions, this method achieves higher-fidelity waveform reconstruction, improving central pulse wave analysis. Importantly, integration into clinical practice is straightforward, leveraging automated, noninvasive brachial cuff measurements already widely used in healthcare. Analyzing cardiac pulse waveforms offers valuable insights into heart health and cardiovascular disease risk, although obtaining the more informative measurements from the central aorta remains challenging due to their invasive nature and limited noninvasive options. To address this, we employed a laboratory-developed cuff device for high-resolution pulse waveform acquisition and constructed a spectral machine learning model to nonlinearly map the brachial wave components to the aortic site. Simultaneous invasive aortic catheter and brachial cuff waveforms were acquired in 115 subjects to evaluate the clinical performance of the developed wave-based approach. Magnitude, shape, and pulse waveform analysis on the measured and reconstructed aortic waveforms were correlated on a beat-to-beat basis. The proposed cuff-based method reconstructed aortic waveform contours with high fidelity (mean normalized-RMS error = 11.3%). Furthermore, continuous signal reconstruction captured dynamic aortic systolic blood pressure (BP) oscillations (r = 0.76, P < 0.05). Method-derived central pressures showed strong correlation with the independent invasive measurement for systolic BP (R2 = 0.83; B [LOA] = −0.3 [−17.0, 16.4] mmHg) and diastolic BP (R2 = 0.58; B [LOA] = −0.7 [−13.1, 11.6] mmHg). Shape-based features are effectively captured by the spectral machine learning method, showing strong correlations and no systemic bias for systolic pressure–time integral (r = 0.91, P < 0.05), diastolic pressure–time integral (r = 0.95, P < 0.05), and subendocardial viability ratio (r = 0.86, P < 0.05). These results suggest that the nonlinear transformation of wave components from the distal to the central site predicts the morphological waveform changes resulting from complex wave propagation and reflection within the cardiovascular network. The proposed wave-based approach holds promise for future applications of noninvasive devices in clinical cardiology.
2024
- Sci. Rep.
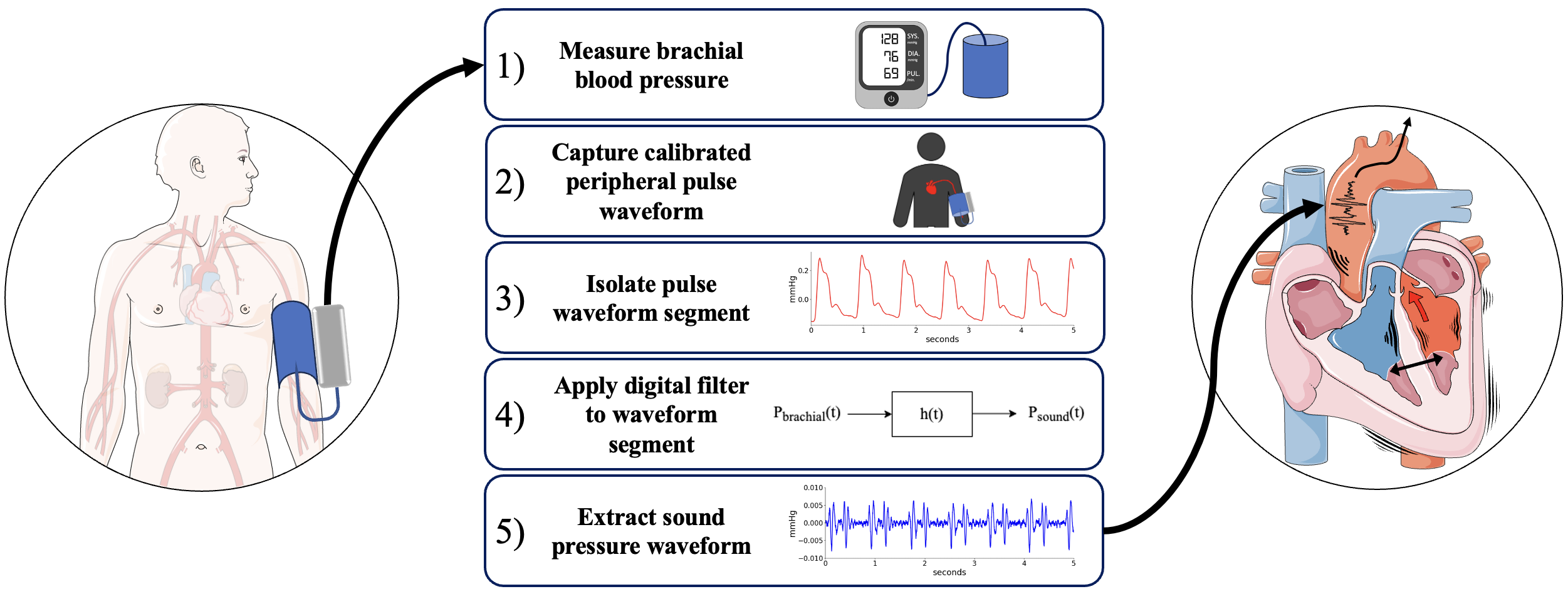 Listening to heart sounds through the pressure waveformAlessio Tamborini, and Morteza GharibSci. Rep., Nov 2024
Listening to heart sounds through the pressure waveformAlessio Tamborini, and Morteza GharibSci. Rep., Nov 2024Non-invasive diagnostic modalities are integral to cardiovascular care; however, current systems primarily measure peripheral pressure, limiting the breadth of cardiovascular prognostication. We report a novel approach for extracting left side heart sounds using a brachial cuff device. The technique leverages brachial cuff device enhanced signal resolution to capture pressure fluctuations generated by cardiohemic system vibrations, the sound pressure waveform. We analyze left heart catheterization data alongside simultaneous brachial cuff device measurements to correlate sound pressure waveform features with left ventricle (LV) contractility. The extracted sound pressure waveform reveals two prominent oscillatory wave packets, termed WP1 and WP2, originating from cardiac structure vibrations associated with LV contractions and relaxation. We demonstrate that WP1 originates from LV contraction during systolic blood ejection through the aortic valve (AV) and is correlated with LV isovolumetric contraction, clinically measured by LV dPdt-max (Pearson-R = 0.65, p < 0.001). Additionally, we show that WP2 comes from LV elongation required for blood flow deceleration at the end of systole, causing AV closure, and is correlated with LV isovolumetric relaxation, measured by LV ndPdt-max (Pearson-R = 0.55, p < 0.001). These findings highlight the value of cuff sound pressure waveforms in providing insights about dynamic coupling of the LV-Aorta complex for non-invasive assessment of LV contractility.
- AbstractAbstract 4141635: Reconstructing invasive aortic pressure waveforms from non-invasive brachial measurements using a machine learning approachAlessio Tamborini, Arian Aghilinejad, and Morteza GharibCirculation, Nov 2024
Background: Pulse wave analysis (PWA) of aortic pressure waveforms carries valuable information about cardiovascular health. However, due to the invasiveness of direct measurement, peripheral pressure waveforms are commonly used as surrogates. Recent findings revealed that while algorithmic methods can preserve pressure dynamics in non-invasive measurements, significant waveform morphology changes disrupt the association between peripheral and central parameters (PMID: 38591330, 2024 ). There is a need for non-invasive methods to faithfully reconstruct the central pressure waveform. Aim: Evaluate the fidelity of reconstructed aortic waveforms using a machine learning method applied to non-invasive brachial measurements. Methods: This study analyzed concurrent aortic catheterization and brachial cuff waveform measurements in suprasystolic mode in subjects referred for non-emergent left heart catheterization (N=115; mean age 66, 63% male). Our machine learning method uses a non-linear mode mapping in the frequency domain between non-invasive calibrated brachial pressure waveforms and invasive aortic ones. PWA features captured waveform magnitude and shape: systolic blood pressure (SBP), diastolic blood pressure (DBP), systolic pressure-time integral (SPTI), diastolic pressure time integral (DPTI), peak timing (T peak ), systole duration (T sys ), maximum pressure derivative ((+)dP/dt), and form factor (FF). Percentage error ((true–predicted)/true) was used to assess parameter correspondence in the testing population (N=35; cardiac cycles=994). Results: The non-linear mapping technique successfully reconstructed central pressure waveforms with good agreement between measured and estimated (MEAN=0.5 mmHg; SD=7.3 mmHg) as shown in Fig. 1A. PWA features showed low mean percentage errors for pressure (SBP=-0.7 \pm5.9%; DBP=-2.9\pm9.4%), area (SPTI=0.2\pm8.7%; DPTI=-2.8\pm9.3%), timing (T peak =1.0\pm12.3%; T sys =1.2\pm7.1%), and shape parameters ((+)dP/dt=11.5\pm24.3%; FF=1.4\pm4.7%) shown in Fig. 1B. Conclusion: Our results demonstrate that the machine learning method applied on brachial cuff measurements captures waveform morphological changes along the arterial system allowing for a faithful representation of the central pressure waveform.
- AbstractAbstract 4142004: Predicting cardiovascular disease events using uncalibrated non-invasive carotid pressure wave components from spectral regression learningArian Aghilinejad, Alessio Tamborini, and Morteza GharibCirculation, Nov 2024
Introduction: Pressure wave separation is a powerful tool for investigating wave phenomena in the cardiovascular system. However, its clinical application has been limited by the need for concurrent measurements of pressure and flow waveforms. Moreover, its predictive value for cardiovascular disease (CVD) remains unclear. Aim: This study aims to assess the performance of pressure wave components for CVD event prediction using single uncalibrated, non-invasive carotid pressure waveforms. Methods: The sample for this study is drawn from the Framingham Heart Study and includes tonometry recordings of carotid pressure waveforms for participants free of CVD at baseline (N=5110; mean age 47 years, 55% women). A recent machine Learning methodology for pressure-only wave separation, called spectral regression learning method ( Eur. Heart J. Open , 2024: oeae040) is applied to decompose the pressure waveform into forward and backward wave components. The pressure wave index, defined as the ratio of the peak backward wave to the sum of the peak forward and backward wave amplitudes, is computed in a testing population (N=1567) blinded to all stages of the regression learning development. Cox proportional hazards regression models and Kaplan-Meier analysis are used in this testing population to relate the pressure wave index to the first incident CVD event (166 cases) over a median follow-up of 9.6 years. Results: The method-derived pressure wave index from uncalibrated carotid pressure waveforms is associated with incident CVD, adjusted for age and sex (model 1; hazard ratio, 0.66 [95% CI, 0.57–0.76] per SD; p Conclusion: Our results demonstrate that the proposed uncalibrated carotid pressure wave index, which can be acquired using affordable non-invasive devices like wearable electronics, is an indicator of vascular health and cardiovascular disease risk.
- Artif Intell Med
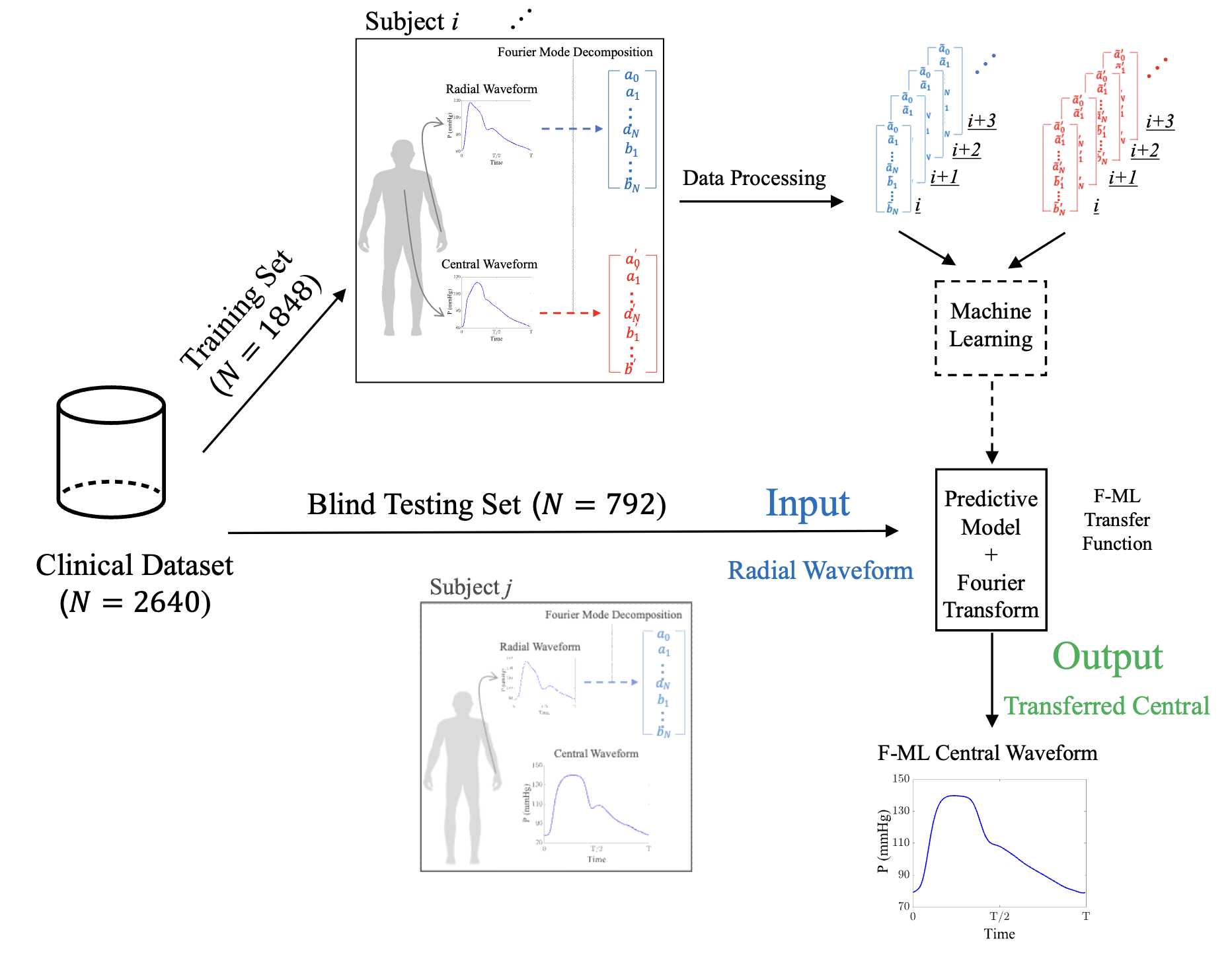 A new methodology for determining the central pressure waveform from peripheral measurement using Fourier-based machine learningArian Aghilinejad, Alessio Tamborini, and Morteza GharibArtif. Intell. Med., Aug 2024
A new methodology for determining the central pressure waveform from peripheral measurement using Fourier-based machine learningArian Aghilinejad, Alessio Tamborini, and Morteza GharibArtif. Intell. Med., Aug 2024Radial applanation tonometry is a well-established technique forhemodynamic monitoring and is becoming popular in affordablenon-invasive wearable healthcare electronics. To assess thecentral aortic pressure using radial-based measurements, thereis an essential need to develop mathematical approaches toestimate the central pressure waveform. In this study, wepropose a new Fourier-based machine learning (F-ML) methodologyto transfer non-invasive radial pressure measurements to thecentral pressure waveform. To test the method, collection oftonometry recordings of the radial and carotid pressuremeasurements are used from the Framingham Heart Study (2640individuals, 55 % women) with mean (range) age of 66 (40-91)years. Method-derived estimates are significantly correlatedwith the measured ones for three major features of the pressurewaveform (systolic blood pressure, r=0.97, p < 0.001; diastolicblood pressure, r=0.99, p < 0.001; and mean blood pressure,r=0.99, p < 0.001). In all cases, the Bland-Altman analysisshows negligible bias in the estimations and error is bounded to5.4 mmHg. Findings also suggest that the F-ML approachreconstructs the shape of the central pressure waveformaccurately with the average normalized root mean square error of5.5 % in the testing population which is blinded to all stagesof machine learning development. The results show that the F-MLtransfer function outperforms the conventional generalizedtransfer function, particularly in terms of reconstructing theshape of the central pressure waveform morphology. The proposedF-ML transfer function can provide accurate estimates for thecentral pressure waveform, and ultimately expand the usage ofnon-invasive devices for central hemodynamic assessment.
- JAHA
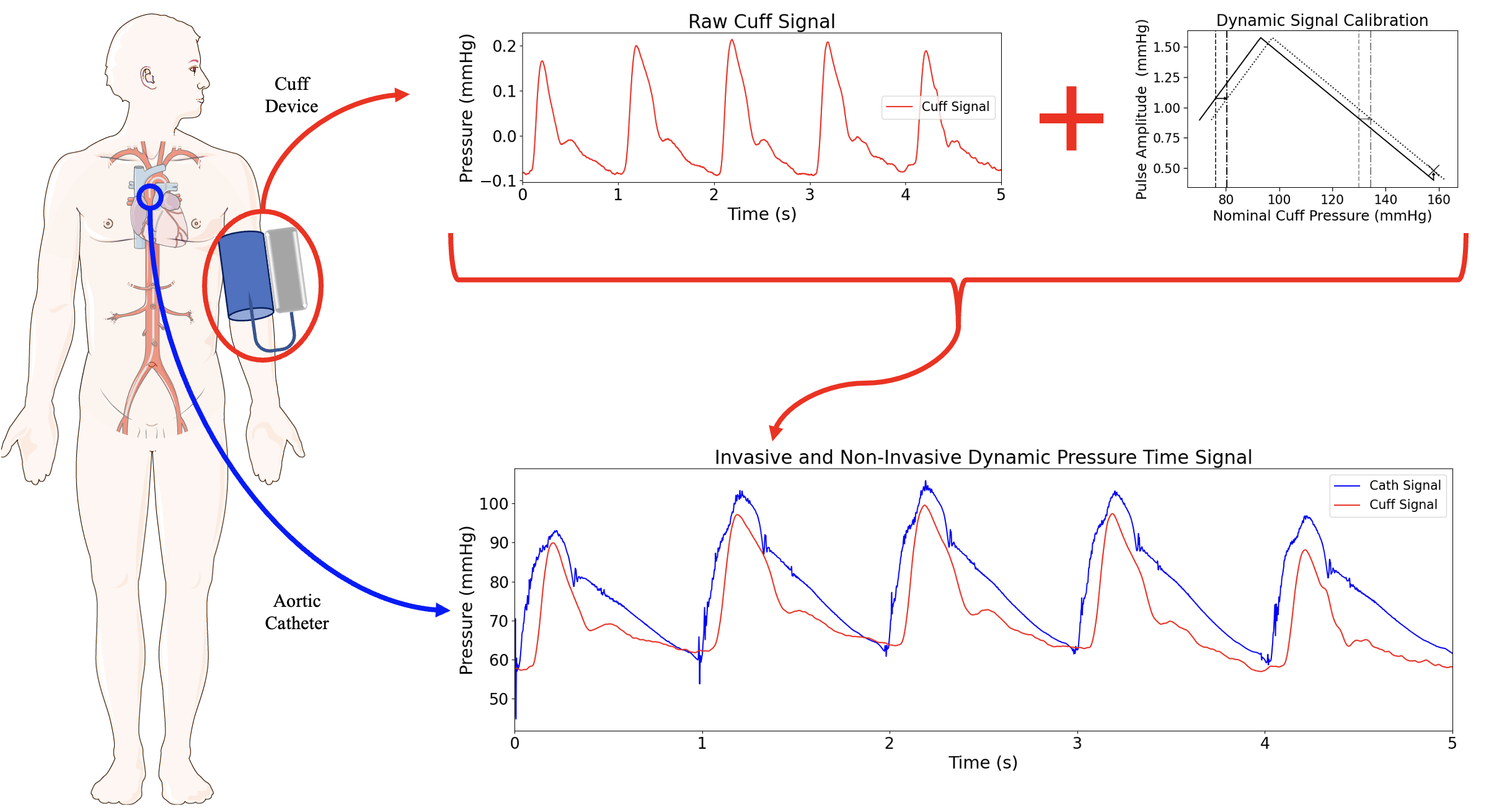 Validation of a suprasystolic cuff system for static and dynamic representation of the central pressure waveformAlessio Tamborini, and Morteza GharibJ. Am. Heart Assoc., Apr 2024
Validation of a suprasystolic cuff system for static and dynamic representation of the central pressure waveformAlessio Tamborini, and Morteza GharibJ. Am. Heart Assoc., Apr 2024BACKGROUND: Noninvasive pulse waveform analysis is valuable forcentral cardiovascular assessment, yet controversies persistover its validity in peripheral measurements. Our objective wasto compare waveform features from a cuff system withsuprasystolic blood pressure hold with an invasive aorticmeasurement. METHODS AND RESULTS: This study analyzed data from88 subjects undergoing concurrent aortic catheterization andbrachial pulse waveform acquisition using a suprasystolic bloodpressure cuff system. Oscillometric blood pressure (BP) wascompared with invasive aortic systolic BP and diastolic BP.Association between cuff and catheter waveform features wasperformed on a set of 15 parameters inclusive of magnitudes,time intervals, pressure-time integrals, and slopes of thepulsations. The evaluation covered both static (subject-averagedvalues) and dynamic (breathing-induced fluctuations) behaviors.Peripheral BP values from the cuff device were higher thancatheter values (systolic BP-residual, 6.5 mm Hg; diastolicBP-residual, 12.4 mm Hg). Physiological correction for pressureamplification in the arterial system improved systolic BPprediction (r2=0.83). Dynamic calibration generated noninvasiveBP fluctuations that reflect those invasively measured (systolicBP Pearson R=0.73, P0.5, P<0.001), encompassing magnitudes,timings, and pressure-time integrals but not slope-basedparameters. CONCLUSIONS: This study demonstrated that the deviceand methods for peripheral waveform measurements presented herecan be used for noninvasive estimation of central BP and asubset of aortic waveform features. These results serve as abenchmark for central cardiovascular assessment usingsuprasystolic BP cuff-based devices and contribute to preservingsystem dynamics in noninvasive measurements.
- PatentMethod for cardiac auscultation using blood pressure cuffAlessio Tamborini, and Morteza GharibUS Patent US20240108303A1, Apr 2024
2023
- PatentSystems, methods, and apparatuses for ocular measurementsMorteza Gharib, Alexei Harvard, Alessio Tamborini, and 1 more authorUS Patent US11839427B2, Dec 2023
- Ann. Biomed. Eng.
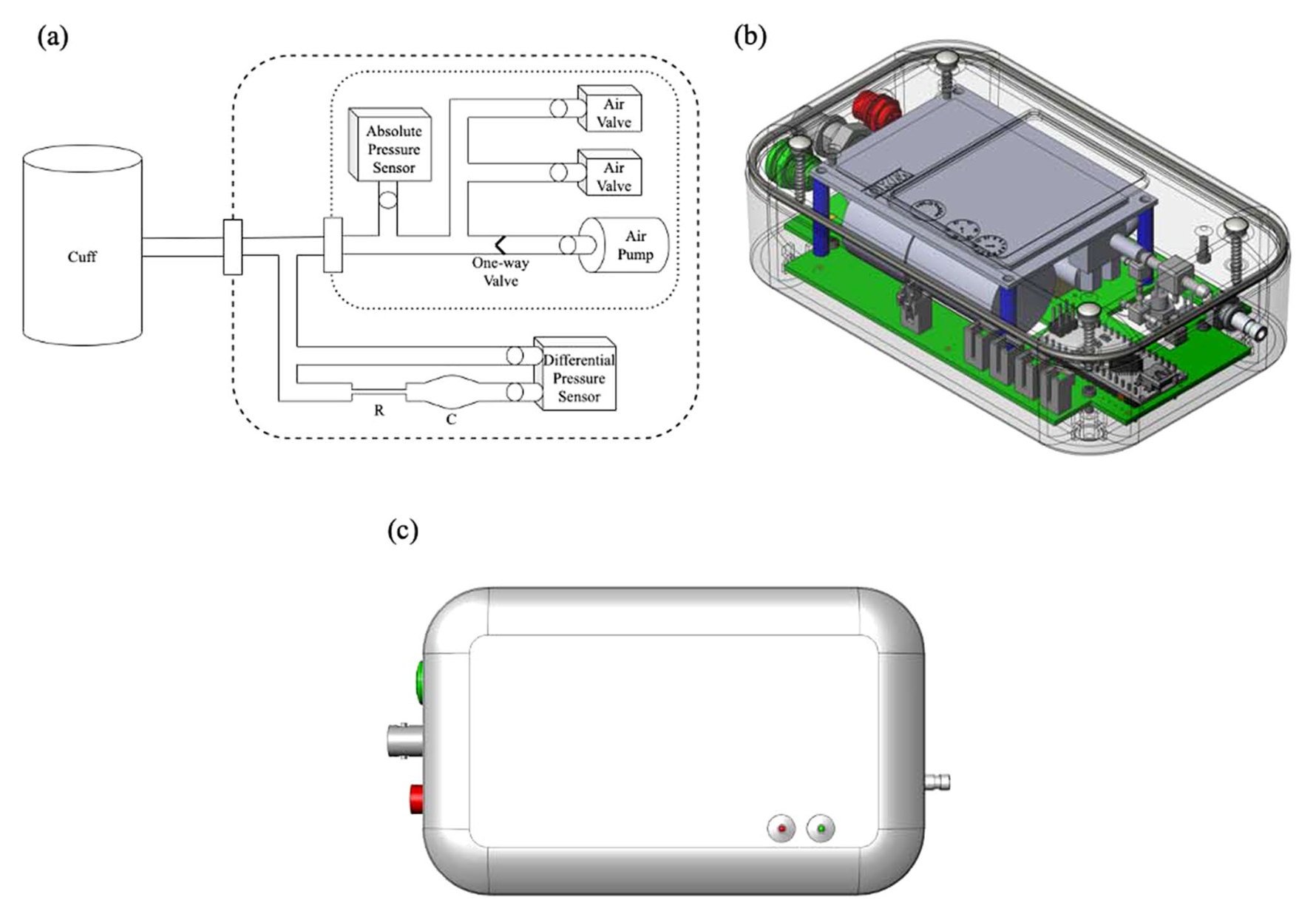 A pneumatic Low-Pass filter for high-fidelity cuff-based pulse waveform acquisitionAlessio Tamborini, and Morteza GharibAnn. Biomed. Eng., Nov 2023
A pneumatic Low-Pass filter for high-fidelity cuff-based pulse waveform acquisitionAlessio Tamborini, and Morteza GharibAnn. Biomed. Eng., Nov 2023Cuff-based pulse waveform acquisition (CBPWA) devices arereliable solutions for non-invasive cardiovascular diagnostics.However, poor signal resolution has limited clinicalapplications. This study aims to demonstrate the improved signalquality of CBPWA devices by implementing passive pneumaticlow-pass filters (pLPF). Conventionally, pressure sensor outputresolution is a percentage of the operating range. Therefore,measurement of small pressure changes in a large range mustsacrifice signal resolution to accommodate for the large meanpressures. We design a pLPF to obtain the running mean pressureand combine it with a high-resolution differential pressuresensor for isolating the signal’s pulsatile component.Thirty-one volunteers participated in a device proof-of-conceptstudy at Caltech. Volunteers were measured at rest in the supineposition on the left arm. The filtering behavior ismathematically modeled and experimentally verified, showing goodagreement between measured and predicted cutoff frequencies. Inthe human study, the device successfully captured high-fidelitypulse waveform measurements for all volunteers: a blood pressure(BP) reading was followed by inflate-and-hold acquisition indiastolic BP (DBP), mean arterial pressure (MAP), and suprasystolic BP (sSBP). The study demonstrated the reliability andhigh signal resolution of pLPF for CBPWA. Considering thewidespread use of the brachial cuff, a system forhigh-resolution CBPWA motivates the clinical implementation ofnon-invasive pulse waveform analysis (PWA).
- PatentSystems and methods for non-invasive pulse pressure waveform measurementAlessio Tamborini, and Morteza GharibUS Patent US20230050058A1, Feb 2023
2019
- Biofabrication
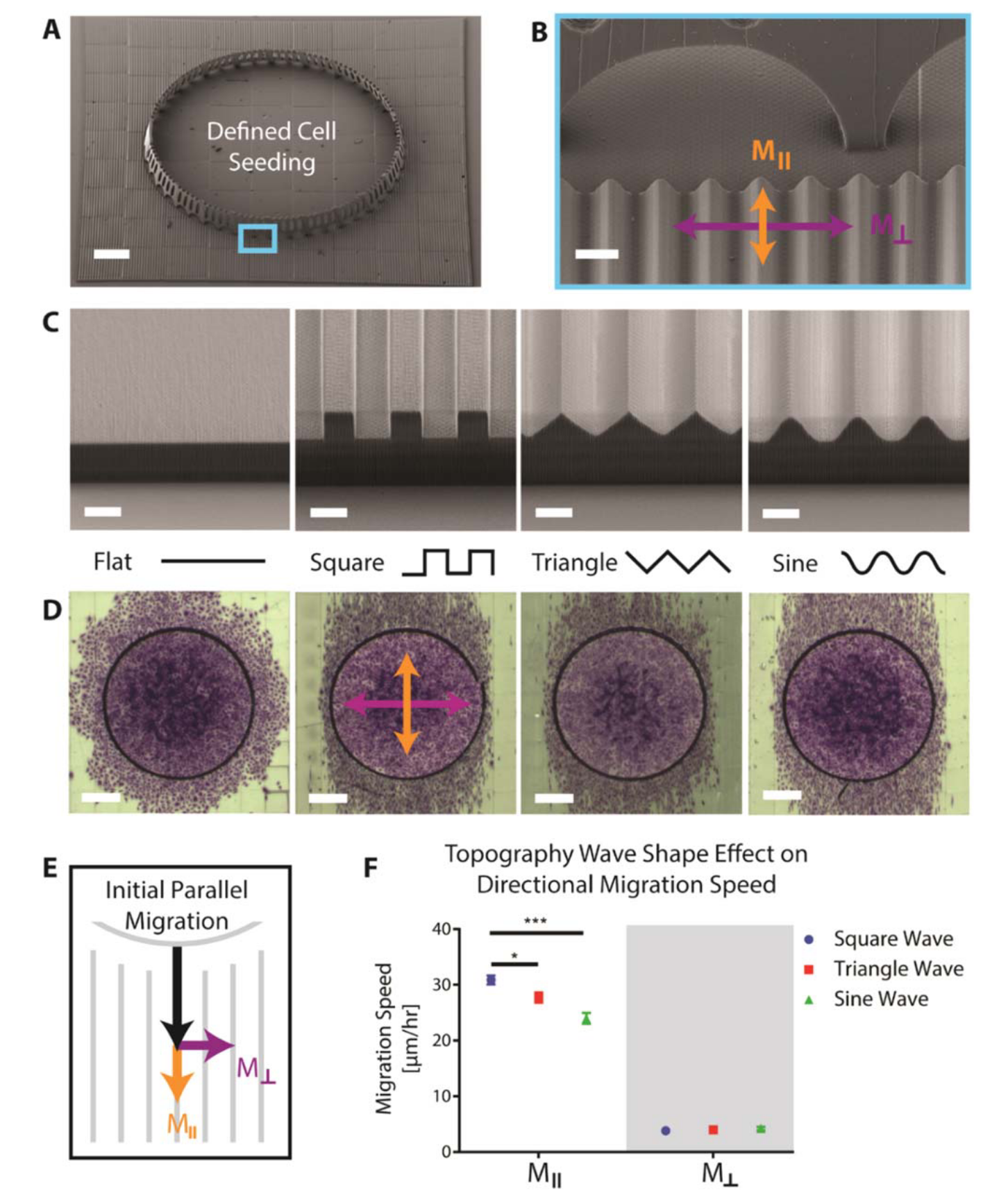 Studies of 3D directed cell migration enabled by direct laser writing of curved wave topographyDaniel Cheng, Rachael K Jayne, Alessio Tamborini, and 3 more authorsBiofabrication, Feb 2019
Studies of 3D directed cell migration enabled by direct laser writing of curved wave topographyDaniel Cheng, Rachael K Jayne, Alessio Tamborini, and 3 more authorsBiofabrication, Feb 2019Cell migration, critical to numerous biological processes, can be guided by surface topography. Studying the effects of topography on cell migration is valuable for enhancing our understanding of directional cell migration and for functionally engineering cell behavior. However, fabrication limitations constrain topography studies to geometries that may not adequately mimic physiological environments. Direct Laser Writing (DLW) provides the necessary 3D flexibility and control to create well-defined waveforms with curvature and length scales that are similar to those found in physiological settings, such as the luminal walls of blood vessels that endothelial cells migrate along. We find that endothelial cells migrate fastest along square waves, intermediate along triangular waves, and slowest along sine waves and that directional cell migration on sine waves decreases as sinusoid wavelength increases. Interestingly, inhibition of Rac1 decreases directional migration on sine wave topographies but not on flat surfaces with micropatterned lines, suggesting that cells may utilize different molecular pathways to sense curved topographies. Our study demonstrates that DLW can be employed to investigate the effects and mechanisms of topography on cell migration by fabricating a wide array of physiologically-relevant surfaces with curvatures that are challenging to fabricate using conventional manufacturing techniques.